Home / Magazine / Process engineering & manufacturing / New ways of creating chemical bonds for increasingly complex molecules
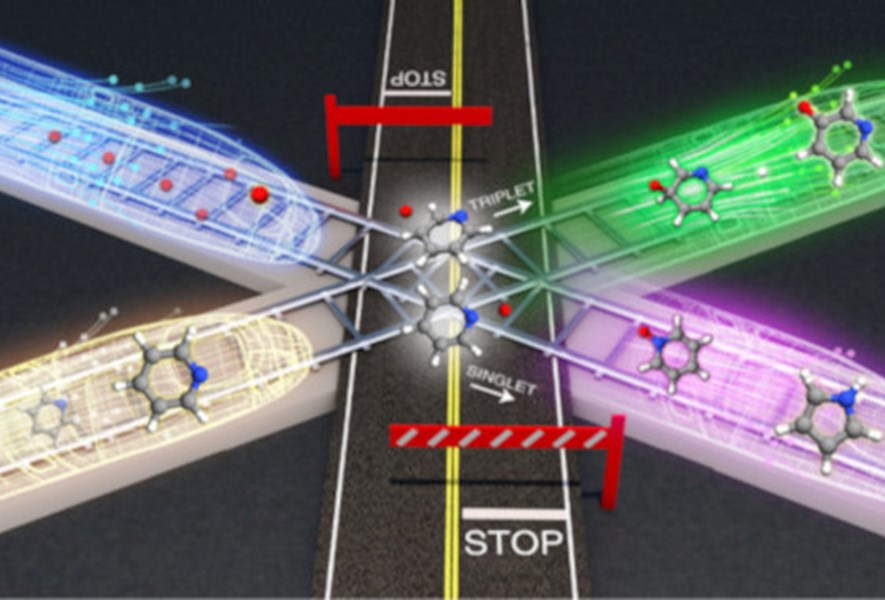
A study published recently in «Nature Chemistry» that combines experimental measurements and theoretical simulations has highlighted a new class of chemical reactions whose speed is controlled by quantum phenomena. Study participants included researchers from the Politecnico di Milano and also from the Universty of Perugia, the Scuola Normale Superiore in Pisa and the University of Bologna.
The discovery can be explained by these images:
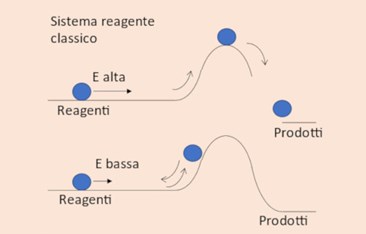
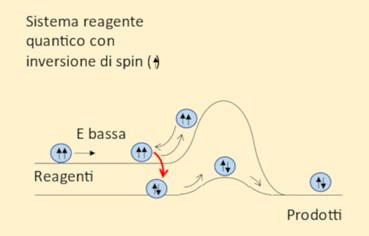
Figure 1 Figure 2
If we imagine a chemical reaction as a pathway that must be travelled in order to go from reactants to products (figure 1), it is reasonable to expect that the reaction will occur if the reactants have sufficient energy to overcome the energy bottleneck (barrier) separating the reactants and the products (top), and that it won’t occur if the energy is insufficient (bottom).
Through the reaction mechanism discovered in this study, we can bypass the barrier even when the reactant’s energy is low, by means of a quantum leap onto a parallel reactive pathway with a lower barrier. The mechanism that enables this reaction is of a quantum nature and is known as spin inversion (figure 2).
«The class of reactions studied, known as spin-forbidden, is difficult to approach because it requires the use of advanced calculation methods» - explains Carlo Alessandro Cavallotti, professor at the Politecnico di Milano and author of the study - «Using new theoretical and computational tools developed by teams in Milan, Pisa and Bologna, we were able to reproduce the experimental data measured by our colleagues in Perugia, demonstrating for the first time that, for a particular reactant system, the addition of oxygen to nitrogen compounds, the proposed mechanism is active».
Evidence of the existence of this new reactive pathway, significant around and below room temperature, will allow us both to identify certain active reaction pathways in astrochemical and biological environments that have hitherto been difficult to understand, and to design new chemical synthesis pathways.
In fact, the proposed reactive pathway allows us to conceive new methods for creating chemical bonds between different reactants and can thus be added to the chemist's toolbox for constructing increasingly complex molecules.
Pedro Recio, Silvia Alessandrini, Gianmarco Vanuzzo, Giacomo Pannacci, Alberto Baggioli, Demian Marchione, Adriana Caracciolo, Vanessa J. Murray, Piergiorgio Casavecchia, Nadia Balucani, Carlo Cavallotti, Cristina Puzzarini & Vincenzo Barone
«Nature Chemistry» (Nat. Chem.) (2022)